Transport Medium(UTM)(COVID-19 sample collection KIT)
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- T-HMS Universal Transport Medium (UTM)
- Payment Terms
- L/C,T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- corona, transport, medium, collecting
Apply a video call to the Supplier

People Medical
- Verified Certificate
-
4

| Product name | Transport Medium(UTM)(COVID-19 sample collection KIT) | Certification | - |
|---|---|---|---|
| Category |
Medical Test Kit
Tongue Depressor Other Examination & Testing Instrumnet |
Ingredients | - |
| Keyword | corona , transport , medium , collecting | Unit Size | - |
| Brand name | T-HMS Universal Transport Medium (UTM) | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 9031 |
Product Information
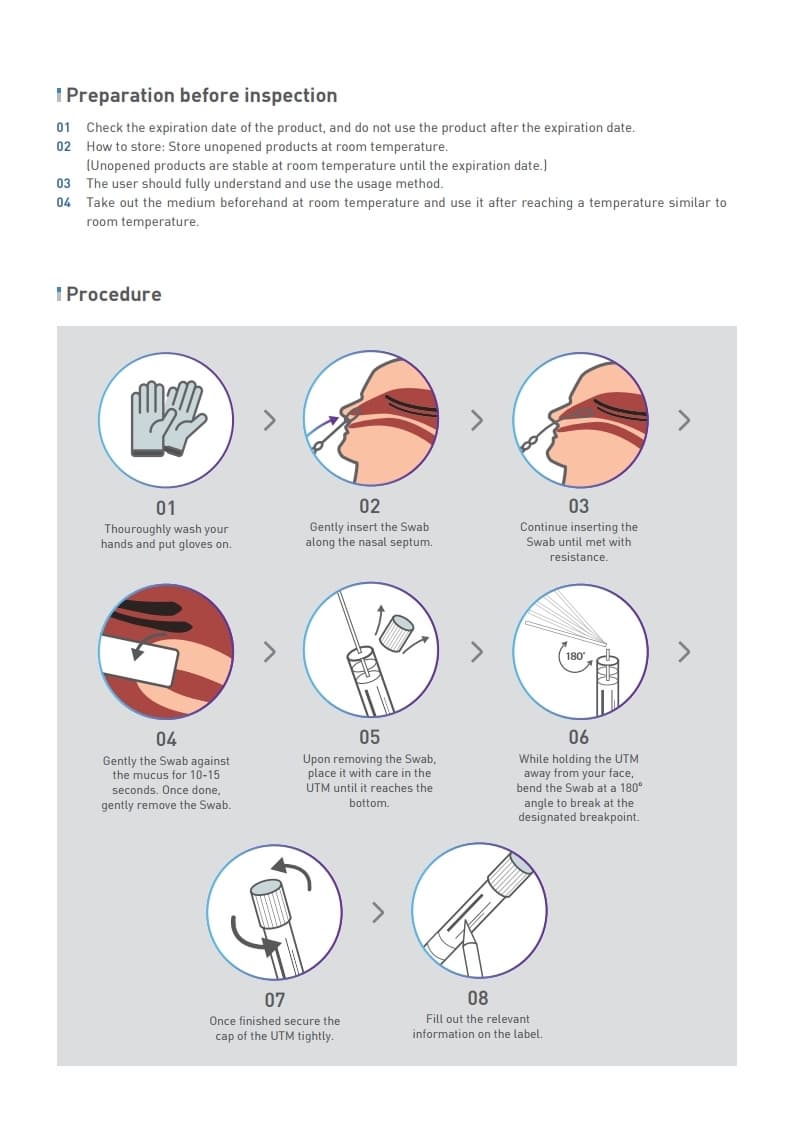
COVID-19 sample collection KIT
코로나-19 검체 채취용 KIT
(T-HMS Universal Transport Medium)
M S D S
MATERIAL SAFETY DATA SHEET
1. PRODUCT AND COMPANY IDENTIFICATION
1.1 Product Name / Model No : COLOR-UTM(Universal Transport Medium) / T-HMS-UTM / 3ml
1.2 Recommended suse of the chemical and restrictions on use : QBIO Universal Transport Medium (UTM) System is intended for the collection and transport of clinical specimens containing viruses,chlamydiae,mycoplasma or ureaplasma from the collection site to the testing laboratory. UTM can be processed using standard clinical laboratory operating procedures for viral, chlamydial, mycoplasma and ureaplasma culture.
1.3 Manufacturer/Supplier/Distributor Information
- Manufacturer -
○ Name : QBIO Co., Ltd.
○ Address : #504, C-Dong, 744, Pangyo-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, Republic of Korea
2. HAZARDS IDENTIFICATION
2.1 Hazard/Risk Classification : None
2.2 Other Hazard/Risk which are not included in the classification criteria : None
3. COMPOSITION/INFORMATION ON INGREDIENTS
Chemical Name | wt% | CAS# |
Sterilization DI-Water | >90 | 7732-18-5 |
Sucrose | <1 | 57-50-1 |
Gelatin | <1 | 9000-70-8 |
HEPES | <1 | 7365-45-9 |
L-glutamic | <1 | 56-86-0 |
Vancomycin | <1 | 1404-93-9 |
Phenol Red | <0.1 | 34487-61-1 |
Amphotericin B | <0.1 | 1397-89-3 |
BSA | <1 | 9048-46-8 |
Hank`s Balanced Salts | <1 |
|
4. FIRST AID MEASURES
4.1 Eye contact : Wash out with plenty of water
4.2 Skin contact : No irritations
4.3 Inhalation : Not dangerous
4.4 Ingestion : Rinse out the mouth and immediately seek medical attention
4.5 Indication of immediate medical attention and notes for physician : No special measures required
5. FIRE-FIGHTING MEASURES
5.1 Suitable (and unsuitable) extinguishing media : Water, foam, powder, CO2
5.2 Specific hazards arising from the chemical : None
5.3 Special protective equipment and precautions for fire-fighters : None
6. ACCIDENTAL RELEASE MEASURES
6.1 Personal precautions, protective equipment and emergency procedures : No necessary
6.2 Environmental precautions and protective procedures : Do not allow product to reach sewage system or any watercourse
6.3 Methods and materials for containment and cleaning up : Absorb liquid components with liquid-binding material and eliminate minor let amounts with water.
7. HANDLING AND STORAGE
7.1 Precautions for safe handling : No special measures required.
7.2 Conditions for safe storage : Should be stored at room temperature (2~30℃) in a tightly closed and lightproof container
7.3 Shelf - life : 1 year 6 month
8. EXPOSURE CONTROLS & PERSONAL PROTECTION
8.1 Control parameters : None
8.2 Appropriate engineering controls : None
8.3 Personal protective equipment : None
9. PHYSICAL AND CHEMICAL PROPERTIES
9.1 Appearance : Clear red liquid
9.2 Odor : Characteristic
9.3 pH (25℃) : 7.4±0.2
9.4 Boiling point : N/A
9.5 Vapor Pressure : N/A
9.6 Specific Gravity : N/A
9.7 Vapor Density) : N/A
9.8 Melting Point : N/A
9.9 Solubility H2O : Complete
9.10 Evaporation Rate : N/A
9.11 Flash Point : N/A
10. STABILITY AND REACTIVITY
10.1 Chemical stability and possibility of hazardous reactions : None
10.2 Conditions to avoid : None
10.3 Incompatible materials : None
10.4 Hazardous decomposition products : None
11. TOZICOLOGICAL INFORMATION
11.1 Information on the likely routes of exposure : There are no toxicological data existing for this product
11.2 Health hazards information
○ Acute toxic : None
○ Skin corrosive/irritant : None
○ Serious eye damage / eye irritations : None
○ Respiratory sensitization : None
○ Skin sensitization : None
○ Carcinogenicity : None
○ Germ Cell Mutagenicity : None
○ Reproductive toxicity : None
○ Specific target organ toxicity (single exposure) : None
○ Specific target organ toxicity (repeated exposure) : None
○ Aspiration hazard : None
12. ECOLOGICAL INFORMATION
12.1 Aquatic and terrestrial ecotoxicity : None
12.2 Persistence and degradability : None
12.3 Bio accumulative potential : None
12.4 Mobility in soil : None
12.5 Other adverse effects : None
13. DISPOSAL CONSIDERATIONS
13.1 Disposal method : Must be disposed in an incinerator
13.2 Disposal precaution : (Including the disposal method of contaminated container and packaging)Disposal must be made according to official regulations.
14. TRANSPORT INFORMATION
The product is not classified.
15. REGULATORY INFORMATION
Labeling as dangerous goods is not required.
16. OTHER INFORMATION
The information herein is given in good faith and corresponding to our present state of knowledge and experience.
The safety data sheet serves as a description of the product in regard to necessary safety measures.
The indications have not the meaning of guarantees on properties.

- Product Info Attached File
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | L/C,T/T | Shipping time | Negotiable |

- President
- Kim Sune
- Address
- 1166,104dong 701 room, Pyeongtaek-si, Gyeonggi-do, Korea
- Product Category
- Medical Test Kit,Other Health Care Products
- Year Established
- 2019
- No. of Total Employees
- 1-50
- Company introduction
-
We, People Medical, will do our best to block infectious diseases with better products and materials for K-POE.Our product is a 100% domestic disposable mask made with Korean manufacturing technology by securing a large amount of domestic fabric.
It passed the tests of the Korea Apparel Testing and Research Institute (KATRI) and the Korea Textile Material Research Institute (KOTERI).
Korea-Prevention Of Epidemics has been filed for a patent.Accordingly, we would like to inform the world of our publicity.
We are pleased to introduce BZ COVID-19 Ag Rapid Test We want to export To many countries
We have received CE certification for the antigen (Ag) test kit among the corona 19 diagnostic test methods in Korea, and we have contacted you to export to Europe, Asia etc
This Corona 19 test kit test (Ag) detects a pharyngeal sample after 3 days, and it is known that the clinical sensitivity is 97% and the specificity is 99.3%.
The manufacturer of this diagnostic kit is http://www.biozentech.co.kr, and the representative of the manufacturer is Professor Chae-Seung Lim, Department of Laboratory Medicine, Korea University Hospital.
- Main Markets
-
 Austria
Austria
 France
France
 Hungary
Hungary
 Spain
Spain
 Sweden
Sweden
 Turkey
Turkey
 U. Kingdom
U. Kingdom
- Main Product
Related Products

Animal Urine Analysis Test Strip Self-Stik VET
BioTracer FOB Rapid Test

PRO PRP KIT
Contour Test strips, Accu chek, One Touch, On Call Plus
Osteopro (X-ray Bone densitometer, DEXA)


































Medium_for_collecting_corona_2.jpg)
Medium_for_collecting_corona_3.jpg)
Medium_for_collecting_corona_4.jpg)
Medium_for_collecting_corona_5.jpg)
Medium_for_collecting_corona_2.jpg)
(COVID-19_sample_collection_KIT)_2.jpg)
 South Korea
South Korea
Medium_for_collecting_corona_2.jpg)
_2.jpg)

